Clinical Laboratory
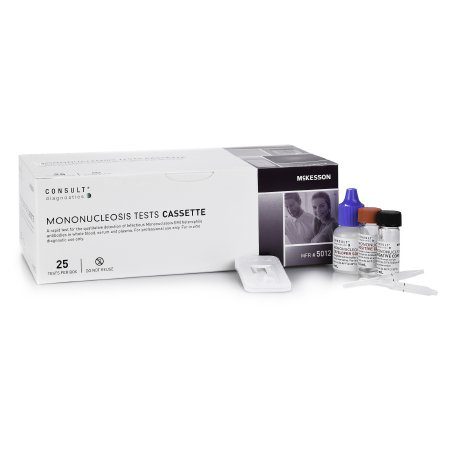
Rapid Test Kit McKesson Consult™ Infectious Disease Immunoassay Infectious Mononucleosis Whole Blood / Serum / Plasma Sample 25 Tests.>#951314
$89.89
| Manufacturer | McKesson Brand |
| Application | Rapid Test Kit |
| CLIA Classification | CLIA Waived for Whole Blood / CLIA Moderately Complex for Serum & Plasma |
| Contents 1 | (25) Cassettes in Sealed Pouches, (25) 10 µL Black Line Sample Transfer Pipettes for use with Serum / Plasma, (25) 25 µL Red Line Sample Transfer Pipettes for use with Whole Blood, 6 mL Developer Solution, 0.5 mL Negative Control, 0.5 mL Positive Control, Instructional insert, Procedure card |
| Number of Tests | 25 Tests |
| Product Dating | McKesson Acceptable Dating: we will ship >= 90 days |
| Reading Type | Visual Read |
| Sample Type | Whole Blood / Serum / Plasma Sample |
| Test Format | Cassette Format |
| Test Name | Infectious Mononucleosis |
| Test Type | Infectious Disease Immunoassay |
| Time to Results | 8 Minute Results |
| UNSPSC Code | 41116126 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Features
- McKesson Consult™ Mononucleosis Cassette Test
- A rapid test for the qualitative detection of Infectious Mononucleosis (IM) heterophile antibodies in whole blood, serum and plasma.
- CLIA Waived for Whole Blood / CLIA Moderately Complex for Serum or Plasma.
- Contents: 25 Cassettes (in sealed pouches), 25 (10 µL) (black line) Sample transfer pipettes for use with serum/plasma, 25 (25 µL) (red line) Sample transfer pipettes for use with whole blood, Developer solution (6 mL; Phosphate saline buffer containing 0.1% Sodium Azide as a preservative), Negative control (0.5 mL; diluted human serum containing 0.1% Sodium Azide as a preservative), Positive control (0.5 mL; diluted human serum containing IM Heterophile Antibodies and 0.1% Sodium Azide as a preservative), Instructional insert, Procedure card
- Results in as fast as eight minutes.
- Greater than 99% accuracy.
- External positive and negative quality controls provided.
- Acute infection determination (IgM Specific Conjugate).
- For in vitro diagnostic use only.
- 18-month shelf life.
- Not made with natural rubber latex.
- Packaged: 25 Tests Per Box
Be the first to review “Rapid Test Kit McKesson Consult™ Infectious Disease Immunoassay Infectious Mononucleosis Whole Blood / Serum / Plasma Sample 25 Tests.>#951314” Cancel reply







Reviews
There are no reviews yet.