Clinical Laboratory
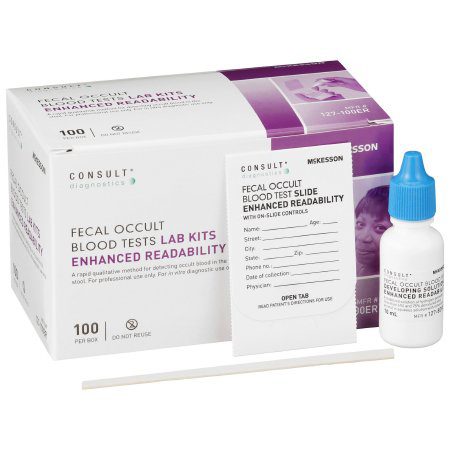
Rapid Test Kit McKesson Consult™ Colorectal Cancer Screening Fecal Occult Blood Test (FOBT) Stool Sample 100 Tests CLIA Waived.> #1060834
$51.32
| Manufacturer | McKesson Brand |
| Application | Rapid Test Kit |
| CLIA Classification | CLIA Waived |
| Contents 1 | (100) Single Slides with On-Slide Controls, (2) Fecal Occult Blood Test Developing Solution, (100) Applicators, Product Instructions |
| Number of Tests | 100 Tests |
| Product Dating | McKesson Acceptable Dating: we will ship >= 90 days |
| Reading Type | Visual Read |
| Sample Type | Stool Sample |
| Technology | Guaiac |
| Test Format | Test Card Format |
| Test Method | Single Developer |
| Test Name | Fecal Occult Blood Test (FOBT) |
| Test Type | Colorectal Cancer Screening |
| Time to Results | 30 Second to 1 Minute Results |
| UNSPSC Code | 41116120 |
| Latex Free Indicator | Not Made with Natural Rubber Latex |
Features
- McKesson Consult™ Fecal Blood Test
- Designed to detect presence of blood in stool specimens
- Guaiac impregnated paper allows for enhanced readability for early detection of even small amounts of blood in the stool
- Results are read after just 30 seconds
- In-office use or take-home test options for patient convenience
- CLIA-waived test requires no specialized training
- If blood is present, blue color quickly develops for easy-to-interpret results
- Slides have two sample sites for improved detection of positive results; as well as positive and negative monitors to help ensure proper performance of the slide and developer
- Offers a more vivid color development for even greater readability
- Instructions are included to help simplify understanding of the test procedure
- USPS compliant packaging is designed to provide safe and efficient specimen transport
- Not made with natural rubber latex.
- Packaged: 100 Per Box
Be the first to review “Rapid Test Kit McKesson Consult™ Colorectal Cancer Screening Fecal Occult Blood Test (FOBT) Stool Sample 100 Tests CLIA Waived.> #1060834” Cancel reply







Reviews
There are no reviews yet.